
Articles
How To Store Blood
Modified: December 7, 2023
Learn how to properly store blood in this informative article. Find out the best practices to ensure its quality and viability for transfusions.
(Many of the links in this article redirect to a specific reviewed product. Your purchase of these products through affiliate links helps to generate commission for Storables.com, at no extra cost. Learn more)
Introduction
Welcome to the world of blood storage, where preserving the life-giving fluid becomes critical for medical purposes. Blood transfusion is a commonly performed procedure that can save lives in various situations, from emergency surgeries to treating chronic conditions. However, to ensure the effectiveness and safety of stored blood, proper storage techniques are essential.
The process of blood storage involves the collection, processing, and preservation of donated blood or its components. It plays a crucial role in maintaining the viability and functionality of blood products until they are ready to be used for transfusion. Improper storage practices can lead to the deterioration of blood components, compromising their quality and potentially posing risks to patients.
In this article, we will explore the importance of proper blood storage, the factors to consider before storage, suitable containers for blood storage, temperature and environmental conditions, guidelines for safe transportation, duration of storage, quality control measures, and the re-infusion and transfusion of stored blood.
Let’s delve deeper into the fascinating world of blood storage and discover how healthcare professionals ensure that the life-giving liquid remains effective and safe for those in need.
Key Takeaways:
- Proper blood storage is crucial for preserving the functional properties of blood components, preventing contamination, optimizing shelf life, and improving patient outcomes. Adherence to guidelines ensures safe and effective transfusions.
- Understanding the duration of blood storage, implementing quality control measures, and following safe transportation guidelines are essential for maintaining the integrity and safety of stored blood. Healthcare professionals play a vital role in ensuring patient safety and positive outcomes.
Read more: How To Store Period Blood
Importance of Proper Blood Storage
Proper blood storage is of utmost importance to maintain the integrity and functionality of blood products. The viability of blood components, such as red blood cells, platelets, and plasma, significantly impacts their effectiveness when transfused into patients. Here’s why proper blood storage is essential:
Preservation of Functional Properties: Blood products contain valuable components that provide specific functions. Red blood cells carry oxygen, platelets aid in blood clotting, and plasma carries vital proteins. Proper storage techniques, including temperature control and suitable containers, help preserve the functional properties of these components, ensuring their efficacy when administered to patients.
Prevention of Contamination: Contamination of blood products can lead to serious complications for patients. By following proper storage protocols, including aseptic techniques and controlled environments, healthcare professionals can minimize the risk of contamination and ensure the safety of patients receiving transfusions.
Optimal Shelf Life: Blood products have a limited shelf life. It is crucial to store them under appropriate conditions to maximize their usability. Proper storage techniques help maintain the quality and viability of blood components, extending their shelf life and allowing for a more efficient use of available resources.
Emergency Preparedness: Hospitals and blood banks must be prepared to respond quickly to emergencies, such as mass casualties or natural disasters. Proper blood storage practices enable healthcare facilities to maintain an adequate supply of blood products and ensure readiness for unexpected events.
Improved Patient Outcomes: Proper blood storage directly contributes to better patient outcomes. By preserving the functionality of blood components, stored blood can effectively treat various medical conditions, including anemia, bleeding disorders, and surgical needs. Ensuring the integrity of stored blood reduces the risk of complications during transfusion, enhancing patient safety and recovery.
In summary, proper blood storage is crucial for preserving the functional properties of blood components, preventing contamination, maximizing shelf life, facilitating emergency preparedness, and improving patient outcomes. Healthcare professionals must adhere to strict guidelines and protocols to ensure that stored blood is safe, effective, and readily available when needed.
Factors to Consider Before Storage
Before storing blood, several important factors must be considered to ensure its preservation and subsequent safe use for transfusion. These factors include:
Donor Suitability:
One of the primary considerations before storing blood is determining the donor’s suitability. Strict donor screening protocols are in place to prevent the transmission of infectious diseases and to ensure the safety of the donated blood. Donors must undergo a thorough medical history assessment and screening tests for diseases that can be transmitted through blood, such as HIV, hepatitis B and C, and syphilis. Only blood from eligible donors is stored for transfusion.
Type and Compatibility:
The blood type and compatibility must be determined before storage to ensure that the right blood products are available for patients when needed. Blood is classified into different types based on the presence or absence of specific antigens on the red blood cells, namely A, B, AB, or O, along with the positive or negative Rh factor. Matching the blood type of the recipient with the appropriate blood product minimizes the risk of adverse reactions during transfusion.
Read more: How To Store Blood In A Vial
Testing and Processing:
Before storing blood, it undergoes a series of rigorous tests to detect infectious diseases and assess its quality. Testing includes screening for blood-borne pathogens, such as HIV, hepatitis, and syphilis, as well as assessing blood cell counts, antibody levels, and clotting factors. Any blood units that do not meet the required standards are discarded. After testing, blood may undergo processing, such as separation into components like red blood cells, platelets, and plasma, to facilitate specific transfusion needs.
Labeling and Documentation:
Accurate labeling and documentation of stored blood units are essential to ensure traceability and prevent errors. Each unit of blood should be labeled with the donor’s identification, blood type, Rh factor, and expiration date. Additionally, important information, such as the date and time of collection, testing, and processing, should be documented in the blood bank records to maintain a comprehensive record of each unit’s storage history.
Storage Conditions:
The storage conditions play a crucial role in maintaining the viability and functionality of blood products. Factors like temperature, lighting, humidity, and ventilation should be carefully regulated within designated storage areas. Blood components may require different storage conditions. For example, red blood cells are typically stored at temperatures between 1°C and 6°C, while platelets are stored at room temperature with gentle agitation to prevent clotting. Following strict guidelines for temperature control and environmental conditions is essential to ensure the quality and safety of stored blood.
By considering these factors, healthcare professionals can ensure the suitability, compatibility, testing, processing, labeling, and appropriate storage of blood units. Attention to detail and adherence to established protocols are vital in maintaining the integrity and safety of stored blood for transfusion.
Suitable Containers for Blood Storage
When it comes to storing blood, selecting the right containers is essential to ensure the preservation of its integrity and functionality. Suitable containers provide a safe and controlled environment that prevents contamination, minimizes exposure to external factors, and maintains the desired storage conditions. Here are some commonly used containers for blood storage:
Read more: How To Store Blood At Home
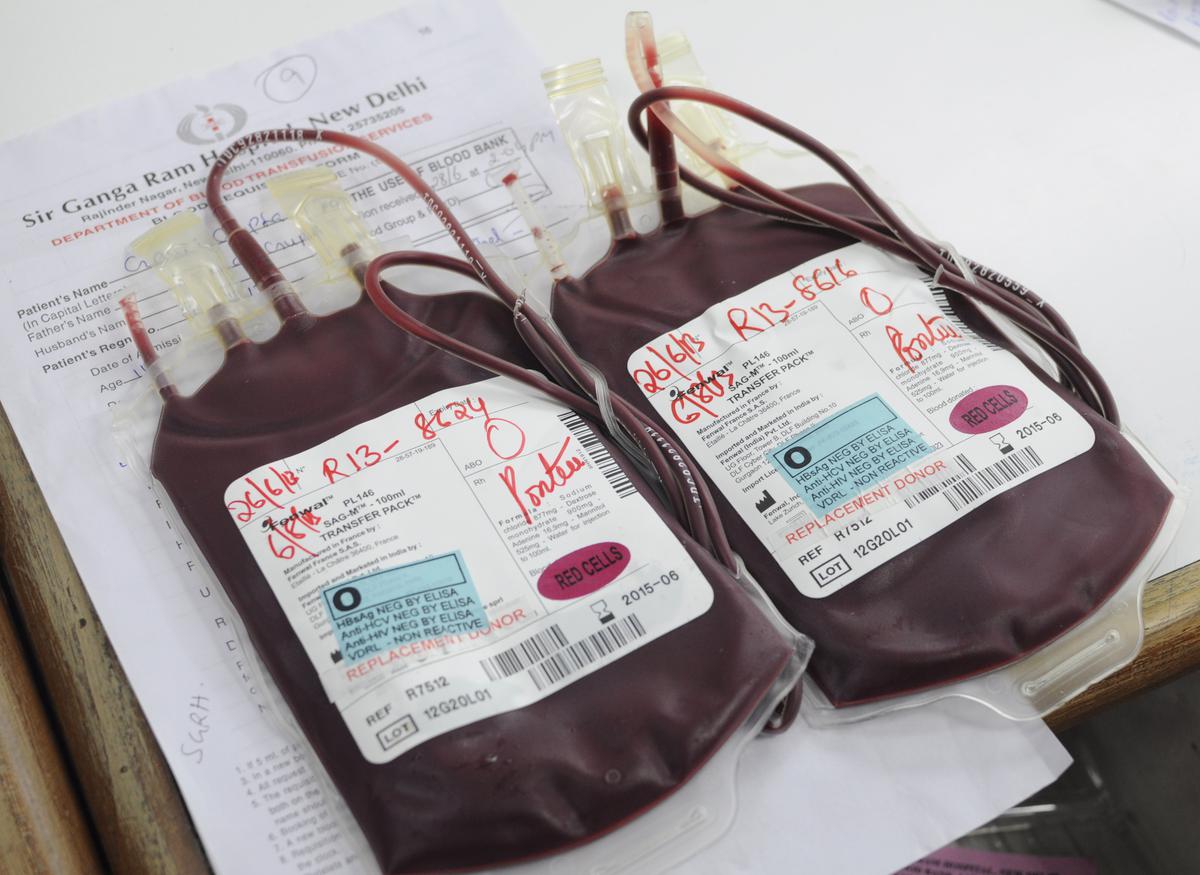
Blood Bags:
Blood bags are a common choice for storing whole blood or its components, such as red blood cells, plasma, or platelets. These specially designed bags are made of medical-grade plastic and are sterile, flexible, and transparent. Blood bags usually come with integrated tubing and ports for easy collection, processing, and transfusion.
Blood bags offer several advantages for blood storage. They are lightweight, space-efficient, and can be easily manipulated during processing and transfusion. The bags also allow for proper labeling and documentation of stored blood units, ensuring traceability and preventing errors.
Collection Tubes:
For smaller blood samples or when collecting blood for laboratory testing, collection tubes are commonly used. These tubes come in various sizes and materials, including glass or plastic, and often contain an anticoagulant to prevent clotting. Once the blood is collected, the tubes can be labeled and transported to the laboratory for analysis.
It is important to note that collection tubes are not suitable for long-term storage of blood components for transfusion. They are primarily used for diagnostic purposes and may not provide the necessary storage conditions to maintain the viability and functionality of blood components over an extended period.
Cryogenic Containers:
For long-term storage of blood components, such as stem cells or rare blood types that require preservation at ultra-low temperatures, cryogenic containers are utilized. These containers are designed to withstand extremely low temperatures, typically below -150°C, and are insulated to maintain a stable storage environment.
Cryogenic containers use liquid nitrogen or other cryopreservative substances to create a freezing environment, ensuring the long-term preservation of blood components. These containers are often equipped with advanced monitoring systems to maintain temperature stability and to provide additional safeguards against temperature fluctuations.
Transportation Containers:
During the transportation of blood from the collection site to the storage facility or the hospital, specialized containers are used to ensure the integrity and safety of the blood products. These containers are typically insulated, shock-resistant, and equipped with temperature control mechanisms to maintain the desired storage conditions.
Transportation containers may include cooling elements, such as ice packs or gel packs, to keep the blood within the required temperature range during transit. They also have proper sealing mechanisms to prevent leaking or contamination during transportation.
In summary, suitable containers for blood storage include blood bags, collection tubes, cryogenic containers, and transportation containers. Each type of container is designed to meet specific storage requirements and ensure the preservation of blood components. By selecting the appropriate containers, healthcare professionals can maintain the integrity and functionality of stored blood, ensuring its safe and effective use for transfusion.
Read more: How To Store Blood Oranges
Temperature and Environment for Blood Storage
The temperature and environment in which blood is stored play a critical role in maintaining the viability and functionality of blood components. Proper temperature control and environmental conditions ensure that the stored blood remains safe and effective for transfusion. Here’s what you need to know about temperature and environment for blood storage:
Temperature:
Temperature control is crucial in preserving the integrity of blood components. The appropriate storage temperatures vary depending on the type of blood product:
- Whole blood: Stored at temperatures between 1°C and 6°C (34°F to 43°F) to preserve the viability of red blood cells;
- Red blood cells: Kept refrigerated at temperatures between 1°C and 6°C (34°F to 43°F) to prevent degradation;
- Platelets: Stored at room temperature (20°C to 24°C or 68°F to 75°F) with gentle agitation to prevent clotting;
- Plasma: Cryoprecipitated plasma is stored at -18°C or colder (-0.4°F or colder), while fresh frozen plasma is stored at -30°C (-22°F) or colder;
- Cryopreserved blood products: Stored at extremely low temperatures, typically below -150°C (-238°F), in cryogenic containers.
Strict temperature control is necessary to prevent the degradation of blood components, which can lead to reduced efficacy or increased risk of adverse reactions during transfusion. Continuous monitoring of storage temperatures is essential to ensure they remain within the appropriate range.
Environmental Conditions:
In addition to temperature control, maintaining the proper environmental conditions is vital for blood storage:
- Lighting: Blood should be stored in areas with low or controlled lighting to minimize exposure to ultraviolet (UV) light. UV light can lead to the breakdown of certain components in blood, resulting in degradation and potential adverse effects during transfusion.
- Humidity: Blood storage areas should maintain appropriate humidity levels, typically between 40% and 60%, to prevent excessive drying or moisture buildup. Extremes in humidity can affect the integrity and functionality of blood components.
- Ventilation: Adequate ventilation is necessary to maintain a clean and controlled environment for blood storage. Proper airflow helps prevent the accumulation of contaminants, such as mold or bacteria, which could compromise the safety and quality of stored blood.
Overall, maintaining the proper temperature and environmental conditions for blood storage is imperative for preserving the viability, functionality, and safety of blood components. Adhering to strict guidelines and regularly monitoring storage areas ensure that blood remains in an optimal state for transfusion, providing the best possible outcomes for patients.
Blood Storage in Hospitals and Blood Banks
Hospitals and blood banks play a crucial role in the storage of blood and blood components, ensuring the availability of safe and effective products for transfusion. These facilities implement strict protocols and guidelines to maintain the quality and integrity of stored blood. Here’s an overview of blood storage in hospitals and blood banks:
Read more: How To Store Blood Worms
Hospitals:
In hospitals, blood storage typically occurs in dedicated transfusion services or blood banks. These specialized areas are equipped with the necessary facilities and equipment to store and handle blood products safely and efficiently. Key aspects of blood storage in hospitals include:
- Storage Areas: Hospitals designate specific areas, such as refrigerators and freezers, solely for blood storage. These areas have temperature monitoring systems in place to ensure that blood products are stored at the appropriate temperatures. Additionally, the storage areas are organized and labeled for easy identification and traceability of blood units.
- Inventory Management: Hospitals maintain careful inventory management to track the availability, expiration dates, and utilization of blood products. This process involves robust tracking systems that help ensure the proper rotation of blood units to prevent wastage due to expired products.
- Transfusion Request Process: When a patient requires a blood transfusion, the hospital’s transfusion service receives a formal request from the healthcare provider. The requested blood products are retrieved from the storage areas, ensuring compatibility with the patient’s blood type and conducting necessary pre-transfusion testing, such as cross-matching, as per standard protocols.
- Transfusion Safety Measures: Before transfusion, hospitals confirm the patient’s identity, ensuring that the blood products are administered to the correct recipient. Additionally, proper labeling and documentation protocols are followed to maintain accurate records of the transfusion, including details such as the blood product used, administration time, and any adverse reactions observed.
Blood Banks:
Blood banks are specialized facilities that collect, test, process, and store donated blood for future use. These banks serve as the primary source of blood supply for hospitals and other healthcare facilities. Here are some fundamental aspects of blood storage in blood banks:
- Donor Screening and Collection: Blood banks follow strict protocols for donor screening and blood collection. Volunteers undergo comprehensive medical history interviews and are tested for specific infectious diseases to ensure the safety of donated blood. Once collected, the blood is appropriately labeled, tested, and processed before storage.
- Storage Facilities: Blood banks have specialized storage facilities that include refrigeration units, freezers, and cryogenic storage systems. These facilities are designed to maintain the required temperatures for different blood components, preserving their viability and functionality over extended periods.
- Inventory Management: Blood banks maintain a comprehensive inventory management system to track donated blood units. This system ensures optimal rotation to prevent wastage and ensures that blood supplies are readily available for hospitals and other healthcare facilities when needed.
- Distribution and Delivery: Blood banks have established processes for the safe transport and delivery of blood products to hospitals and other healthcare facilities. Temperature-controlled transportation systems, proper packaging, and adherence to regulatory guidelines minimize the risk of temperature fluctuations and contamination during transit.
- Quality Control: Blood banks conduct regular quality control measures to ensure the safety and efficacy of stored blood. These measures include routine testing, monitoring storage conditions, and adherence to regulatory standards to maintain the highest quality standards for blood products.
Both hospitals and blood banks adhere to strict protocols and guidelines to maintain the safety and efficacy of stored blood products. By following standardized procedures for storage, inventory management, and transfusion, healthcare professionals ensure that patients receive the safest and most effective blood components when needed.
Guidelines for Safe Transportation and Delivery of Stored Blood
The transportation and delivery of stored blood is a critical step in ensuring that blood products are safely delivered to hospitals and healthcare facilities for transfusion. Following specific guidelines and best practices helps maintain the integrity, safety, and efficacy of stored blood during transit. Here are some essential guidelines for the safe transportation and delivery of stored blood:
Temperature Control:
Maintaining proper temperature control during transportation is crucial to preserve the viability and functionality of blood products. Here are some key considerations:
- Use Temperature-Controlled Containers: Blood products should be transported in containers that can maintain the desired temperature range. Insulated coolers or refrigerated containers equipped with temperature monitoring devices help ensure that the blood remains within the appropriate temperature range.
- Utilize Cooling Elements: Include cooling elements, such as ice packs or gel packs, to help maintain the required temperature during transit. These cooling elements should be appropriately wrapped or packed to avoid direct contact with the blood products.
- Avoid Extreme Temperature Fluctuations: Minimize exposure to extreme temperatures, both hot and cold, as this can compromise the viability and functionality of blood components. Transporting blood in temperature-controlled vehicles or refrigerated transport systems is highly recommended.
Read more: How To Store Blood Samples
Proper Packaging and Labeling:
The packaging and labeling of blood products during transportation are essential to maintain traceability and prevent contamination. Follow these guidelines:
- Ensure Appropriate Packing Materials: Use leak-proof and puncture-resistant packaging materials to prevent spillage and contamination during transit. Secure blood bags, collection tubes, or cryogenic containers inside the packaging to minimize movement.
- Proper Labeling of Containers: Clearly label containers with important information, such as the blood type, donor identification, expiration date, and any special storage requirements. Barcoding or other identification systems can aid in ensuring accurate tracking and traceability.
- Document Transportation Details: Maintain proper documentation of the transportation process, including the date and time of departure, expected arrival, and the responsible individuals involved. This documentation helps track the chain of custody and ensures accountability.
Adherence to Regulatory Standards:
Complying with regulatory guidelines and standards is crucial for the safe transportation and delivery of blood products:
- Follow Transportation Regulations: Familiarize yourself with local and international transportation regulations specific to the transportation of biological substances, including blood products. Adhere to the guidelines concerning packaging, labeling, documentation, and temperature control.
- Training and Certification: Ensure that personnel involved in the transportation and delivery of blood products receive appropriate training and certification. Proper training helps minimize the risk of handling errors, contamination, and temperature deviations during transit.
- Contingency Plans: Develop contingency plans in case of transportation delays, emergencies, or unforeseen circumstances. This ensures that appropriate measures are in place to maintain the integrity and safety of blood products during transit.
By following these guidelines for safe transportation and delivery, healthcare professionals can ensure that stored blood products arrive at their destination in optimal condition. Proper temperature control, packaging, labeling, and adherence to regulatory standards play crucial roles in maintaining the safety and efficacy of blood products, ultimately benefiting patients in need of transfusion.
Duration of Blood Storage and Expiration Dates
The duration of blood storage is a critical aspect that determines the viability and safety of blood products for transfusion. Every blood unit has an expiration date, beyond which the quality and efficacy of the components may be compromised. Understanding the duration of blood storage and interpreting expiration dates is essential in ensuring the use of safe and effective blood products. Here’s what you need to know:
Expiration Dates:
Each blood unit is assigned an expiration date, indicating the date until which the blood is considered viable for transfusion. The expiration date is determined based on factors such as the storage conditions, blood component type, and specific regulatory guidelines. It is essential to adhere to these expiration dates to guarantee the safety and effectiveness of blood products.
Hospitals and blood banks must carefully monitor and manage blood inventory to ensure that no blood units are used beyond their expiration dates. Expired blood products should be discarded following proper protocols to avoid any potential risks to patients.
Read more: How To Store Your Own Blood
Duration of Blood Storage:
The duration of blood storage depends on the specific blood component and its intended use. Here are some general guidelines for the storage durations of common blood components:
- Whole Blood: Typically stored for up to 35 to 42 days, depending on the anticoagulant or preservative used.
- Red Blood Cells: Red blood cells can be stored for up to 42 days under proper refrigeration conditions.
- Platelets: Platelets have a shorter storage duration and are typically stored at room temperature with gentle agitation to prevent clotting. They can be stored for up to 5 to 7 days.
- Plasma: Fresh frozen plasma can be stored for up to 1 year when kept at -30°C (-22°F) or colder. Cryoprecipitated plasma has a shorter storage duration of 1 year at -18°C (0.4°F) or colder.
It is important to note that these are general storage durations, and specific guidelines may vary depending on regulatory requirements and blood bank policies. Proper temperature control and adherence to storage protocols are crucial in maintaining the quality of blood components throughout their storage duration.
Inventory Management and Rotation:
Effective inventory management and rotation techniques are crucial to ensure that blood units are transfused before reaching their expiration dates. The first-in, first-out (FIFO) principle is commonly employed, ensuring that the oldest blood units are used first to minimize wastage due to expired products.
Regular monitoring of blood inventory and close coordination between the transfusion service and blood bank personnel help prevent the use of expired blood units. It is essential for healthcare professionals to be well-versed in interpreting expiration dates and adhering to established procedures to ensure patient safety.
In summary, the duration of blood storage varies based on the blood component type, storage conditions, and regulatory guidelines. Adherence to expiration dates and proper inventory management are crucial in ensuring the safety and efficacy of blood products. By closely monitoring storage durations and implementing proper rotation procedures, healthcare professionals can optimize blood utilization and ensure the availability of safe and effective blood units for transfusion.
Quality Control Measures for Stored Blood
Ensuring the quality and safety of stored blood is of utmost importance in the field of transfusion medicine. Quality control measures are implemented at various stages to monitor and maintain the integrity of blood products throughout their storage. These measures help identify potential issues and ensure that only safe and effective blood is used for transfusion. Here are some key quality control measures for stored blood:
Visual Inspection:
Visual inspection is a fundamental quality control measure for stored blood. This includes regular assessments of blood bags, collection tubes, or other containers for any signs of leakage, discoloration, or abnormalities. Any visually compromised blood units should be removed and discarded according to proper protocols to prevent the use of potentially compromised products.
Read more: How To Get A Blood Stain Out Of A Carpet
Temperature Monitoring:
Continuous temperature monitoring is critical to maintaining the appropriate storage conditions for blood products. Temperature monitoring devices, such as data loggers or digital thermometers, are used to measure and record temperature variations within storage areas. These devices provide real-time data for healthcare professionals to ensure that the blood is stored within the recommended temperature range.
Testing for Infectious Diseases:
To ensure the safety of stored blood, rigorous testing for infectious diseases is performed. Blood samples are screened for pathogens such as HIV, hepatitis B and C, syphilis, and other relevant infectious agents. These tests are conducted at the time of donation and, in some cases, repeated during the storage period to detect any potential infections that may have arisen post-collection. Blood units that test positive for infectious agents are promptly discarded to prevent the transmission of diseases to recipients.
Quality Control of Blood Components:
Blood components, such as red blood cells, platelets, or plasma, undergo regular quality control testing to assess their viability and functionality before transfusion. These tests may include assessing cell counts, hemoglobin levels, clotting factors, or other important parameters, depending on the specific blood product. Ensuring that blood components meet established quality standards helps minimize the risk of adverse reactions and maximizes their effectiveness during transfusion.
Proper Documentation and Traceability:
Accurate documentation and traceability of blood products are essential quality control measures. Proper labeling of blood units with important information, including donor identification, blood type, storage duration, and expiration date, ensures traceability and facilitates proper inventory management. Detailed records of testing results, storage conditions, and any deviations from the recommended protocols should also be maintained to provide a comprehensive history of each blood unit.
Read more: How To Get Blood Out Of A White Blanket
Regular Audits and Inspections:
Regular audits and inspections are conducted to assess compliance with quality control measures. These audits may be conducted internally within the blood bank or externally by regulatory bodies to ensure adherence to established guidelines and protocols. The audits assess the overall processes, documentation, storage conditions, and quality control procedures to identify areas for improvement and ensure the highest standard of quality for stored blood.
By implementing these quality control measures, healthcare professionals can ensure the safety, efficacy, and traceability of stored blood products. Regular inspections, temperature monitoring, testing for infectious diseases, quality control testing of blood components, and proper documentation play key roles in maintaining the integrity and safety of blood throughout its storage, ensuring the best possible outcomes for patients receiving transfusions.
Re-infusion and Transfusion of Stored Blood
The re-infusion and transfusion of stored blood play vital roles in medical interventions, emergency care, and surgical procedures. Proper handling and administration of stored blood are essential to ensure patient safety and optimize the benefits of transfusion. Here’s what you need to know about re-infusion and transfusion of stored blood:
Re-infusion of Autologous Blood:
Re-infusion refers to the process of re-introducing a patient’s own blood, collected and stored prior to a medical procedure, back into their body. It is commonly used in surgical settings, particularly for procedures associated with anticipated blood loss. Some important aspects of re-infusion include:
- Pre-Procedure Collection: Before the procedure, the patient’s blood is collected and stored under controlled conditions. This can be done using techniques such as pre-deposit autologous blood donation or intraoperative cell salvage.
- Effective Storage: The collected blood is stored in suitable containers and maintained at the appropriate temperature to preserve its functionality and integrity until re-infusion.
- Compatibility Check: Before re-infusion, the collected blood is cross-matched with the patient’s current blood type and screened for any infectious or pathogenic agents. This step ensures compatibility and reduces the risk of adverse reactions.
- Re-infusion Process: The stored blood is typically re-infused into the patient during or after the procedure, using sterile techniques and specialized infusion equipment. Healthcare professionals closely monitor the patient for any potential complications.
Transfusion of Stored Blood:
Transfusion refers to the process of delivering blood or blood components from a donor to a recipient. This procedure is commonly performed to restore blood volume, improve oxygen-carrying capacity, or provide specific blood components to patients in need. Key considerations for transfusion of stored blood include:
- Compatibility Assessment: Prior to transfusion, compatibility between the donor blood and recipient is determined through blood type and Rh factor matching. Compatibility is crucial to minimize the risk of adverse reactions.
- Selection of Blood Components: Depending on the patient’s specific needs, different blood components may be selected for transfusion. Commonly transfused components include red blood cells, platelets, plasma, or cryoprecipitate, each serving specific therapeutic purposes.
- Storage Duration Consideration: Stored blood has a limited shelf life, and the duration of storage impacts its efficacy. Healthcare professionals ensure that the transfused blood units have not exceeded their expiration dates, maximizing the benefits and minimizing the risks associated with older blood products.
- Administration Process: The transfusion process involves carefully administering the stored blood or blood components, following established protocols and transfusion guidelines. Adequate monitoring, including the patient’s vital signs, helps identify any adverse reactions or complications promptly.
Throughout the re-infusion and transfusion processes, patient safety is a top priority. Healthcare professionals follow strict protocols, handle the stored blood with care, and closely monitor patients for any signs of adverse reactions. Documentation of the transfusion process is crucial for accurate record-keeping and traceability.
By adhering to established guidelines, healthcare professionals can ensure successful re-infusion and transfusion of stored blood, providing patients with the necessary support and potentially lifesaving interventions in a variety of medical conditions and situations.
Read more: How To Get Blood Out Of Clothes
Conclusion
The storage of blood is a critical process that directly impacts the safety, quality, and efficacy of blood products used for transfusion. Proper blood storage techniques ensure that blood components retain their functionality and viability, providing patients with the best possible outcomes. Throughout this article, we have explored various aspects of blood storage, including the importance of proper storage, factors to consider before storage, suitable containers, temperature and environmental requirements, blood storage in hospitals and blood banks, guidelines for safe transportation and delivery, duration of storage and expiration dates, quality control measures, and re-infusion and transfusion of stored blood.
Proper blood storage practices are essential for preserving the functional properties of blood components, preventing contamination, optimizing shelf life, facilitating emergency preparedness, and improving patient outcomes. Donor suitability, compatibility assessment, testing and processing, and proper labeling and documentation are key factors to consider before storing blood. Suitable containers such as blood bags, collection tubes, and cryogenic containers maintain the necessary storage conditions. Temperature control and environmental conditions play crucial roles in maintaining blood viability. Both hospitals and blood banks are responsible for safe blood storage, ensuring proper inventory management, accurate transfusion requests, and compliance with regulatory standards.
Guidelines for the safe transportation and delivery of stored blood emphasize temperature control, proper packaging, labeling, and adherence to regulatory standards. Understanding the duration of blood storage and interpreting expiration dates ensures the safe use of blood products. Quality control measures such as visual inspection, temperature monitoring, testing for infectious diseases, and documentation and traceability help maintain the integrity of stored blood. Lastly, re-infusion of autologous blood and transfusion of stored blood rely on compatibility assessments and adherence to transfusion protocols to ensure patient safety.
In conclusion, the storage of blood is an intricate and vital process in the field of transfusion medicine. By following proper storage guidelines and adhering to established protocols and quality control measures, healthcare professionals can ensure the safety, efficacy, and traceability of stored blood products. Ultimately, these efforts contribute to improved patient outcomes and provide a lifeline for those in need of blood transfusions.
Frequently Asked Questions about How To Store Blood
Was this page helpful?
At Storables.com, we guarantee accurate and reliable information. Our content, validated by Expert Board Contributors, is crafted following stringent Editorial Policies. We're committed to providing you with well-researched, expert-backed insights for all your informational needs.







0 thoughts on “How To Store Blood”